A study of anthocyanin self-association by NMR spectroscopy†
Abstract
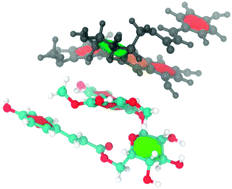
Research involving the development of anthocyanin-containing food colorants has led to the discovery that acylated anthocyanins exhibit remarkable stability, with this improved stabilization being attributed to intra and inter-molecular association. In this work the self-association behavior (intra- and inter-molecular association) of malvidin-3-O-(6-coumaroylglucoside) (mv3cumglc) and malvidin-3-O-glucoside (mv3glc) in the flavylium form (pH 1.0) was studied using a synergistic combination of NMR and molecular dynamics simulation. NOESY experiments have suggested the occurrence of intra-molecular association on malvidin-3-O-coumaroylglucoside with the coumaric acid residue stacking in the anthocyanidin nucleus. The NMR spectral variations (chemical shifts, δ, and diffusion coefficients, D) resulting from the increase of the anthocyanin concentration were analyzed using an isodesmic model. In D2O–DMSO mixtures and in the concentration range tested, mv3cumglc was found to self-associate with a higher affinity constant (1402 M−1 (δ) and 2203 M−1 (D)) compared to mv3glc (819 M−1 (δ) and 1002 M−1 (D)) revealing that in acylated anthocyanins the combined aggregation phenomenon could be responsible for this higher affinity towards self-association. In D2O–DMSO the critical aggregation concentration (cac) values determined for both anthocyanins were very similar, though this value was lower for mv3cumglc.

 Please wait while we load your content...
Please wait while we load your content...