Versatile O- and S-functionalized 1,2,3-triazoliums: ionic liquids for the Baylis–Hillman reaction and ligand precursors for stable MIC-transition metal complexes†
Abstract
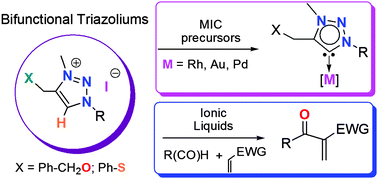
The efficient synthesis of O- and S-functionalized 1,2,3-triazoliums is reported. Owing to their physical properties, these cations are efficient ionic liquids for Baylis–Hillman addition under mild reaction conditions. Simultaneously, the functionalization of the triazolium rings allows for the in situ C-5 metallation providing air stable triazol-5-ylidene Rh(I) Au(I), and Pd(II) complexes. The present work constitutes a rare example of versatile triazolium salts capable of serving in two unrelated synthetic procedures.

 Please wait while we load your content...
Please wait while we load your content...