NMR, DFT and luminescence studies of the complexation of V(v) oxoions in solution with 8-hydroxyquinoline-5-sulfonate†
Abstract
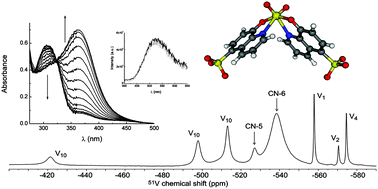
1H, 13C and 51V NMR spectra, DFT calculations, and UV/visible absorption and luminescence spectra are used to study complexation of 8-hydroxyquinoline-5-sulfonate (8-HQS) with vanadium(V). A full speciation study over the concentration region studied shows that V(V) oxoions form three complexes in the pH range 4–7. Geometries are proposed based on NMR and DFT calculations. Dominant species are 1 : 2 (metal : ligand) mononuclear six-coordinated isomers with an almost octahedral geometry, having the metal centre coordinated to two 8-HQS ligands, and only differing in the arrangement of the donor groups. A minor binuclear complex species was also identified, and possesses two five coordinated metal centres, with distorted trigonal bipyramidal geometry, bridged by two coordinated 8-HQS molecules. Upon binding to V(V), marked changes are seen in UV/visible absorption spectra. However, in contrast to other metals (Al(III), Ga(III), Zn(II)), the complexes are only weakly luminescent; this is suggested to be due to quenching of the emission by low-lying ligand-to-metal (LMCT) states close to the emitting ligand-based excited state.

 Please wait while we load your content...
Please wait while we load your content...