Synthesis and characterization of new triphenylamino-1,8-naphthalimides for organic light-emitting diode applications†
Abstract
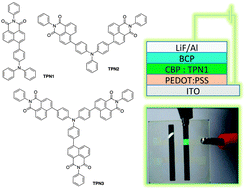
Three new triphenylamino naphthalimides (TPN1–3) were successfully synthesized using Suzuki cross-coupling reactions between N-phenyl-1,8-naphthalimide and triphenylamine precursors. In CHCl3 solution phase, their maximum absorption wavelengths are around 430–433 nm, while the maximum emission wavelength was at 564–599 nm with quantum yields in the range of 0.34–0.55 in CHCl3. In a solid thin-film state, where the packing force enhances the conjugated system and prevents molecular vibration, their absorption bands move towards the longer wavelengths and the emission peaks shift to shorter wavelengths. These compounds show excellent thermal stabilities with their 10% weight loss temperatures well above 350 °C. The results from the electrochemical investigation using cyclic voltammetry agrees with the data from computational calculations using the Gaussian 09 program with B3LYP/6-31G(d,p) geometry optimization. When the multi-layered OLED device with a ITO/PEDOT:PSS/TPN1:CBP/BCP/LiF/Al structure was fabricated, a maximum brightness of 10 404 cd m−2 of yellowish green light at an applied voltage of 19 V and turn-on voltage of 5.8 V was observed. The effect of the solubility of these compounds on device performance was confirmed by the AFM images of the spin-casted thin films.

 Please wait while we load your content...
Please wait while we load your content...