Polarity-tunable and wavelength-tunable bacteriochlorins bearing a single carboxylic acid or NHS ester. Use in a protein bioconjugation model system†
Abstract
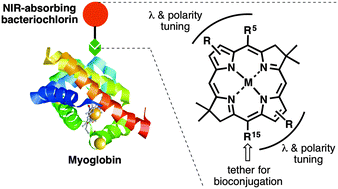
To broaden the scope of near-infrared (NIR)-active chromophores available for bioconjugation with proteins, 10 new bacteriochlorins have been synthesized: six are lipophilic and bear a carboxylic acid tether; four are hydrophilic and bear four carboxylic acids and one N-hydroxysuccinimido (NHS) ester tether. Each bacteriochlorin exhibits a sharp long-wavelength absorption (Qy) band in the NIR region (727–823 nm). The lipophilic bacteriochlorins were examined in DMF (fluorescence quantum yield Φf = 0.037–0.19) whereas the hydrophilic bacteriochlorins were examined in DMF (Φf also = 0.037–0.19) or aqueous phosphate buffer (Φf = 0.0011–0.13). Two bacteriochlorins were conjugated to myoglobin (Mb), which contains ∼14 accessible amino groups. Use of 2, 10, or 50 equivalents of a hydrophilic bacteriochlorin–NHS ester (BC7) gave average loadings of 0.62, 1.6, or 7.1 bacteriochlorins/Mb as determined by absorption spectral comparison with the strongly absorbing heme ligand. MALDI-MS analysis showed a distribution of 0–9 bound bacteriochlorins for the conjugate sample with average loading of 7.1. The Mb–BC7 conjugates exhibited characteristic absorption and fluorescence spectra in aqueous buffer, yet the Φf value was markedly low (Φf ∼ 0.02) regardless of loading versus that of the BC7 monomer (Φf = 0.12), attributed in part to heme quenching. Removal of the heme revealed a loading-dependent Φf, which ranged from 0.091 (0.62 loading) to 0.023 (7.1 loading). The decrease in Φf with increased loading is attributed to self-quenching perhaps facilitated by excited-state energy transfer among the bacteriochlorins (Förster R0 = 59 Å). Taken together, the results show facile access to a collection of useful bacteriochlorins for NIR spectroscopic studies, along with a pigment–protein system that serves the dual purposes of a convenient testbed for evaluating protein bioconjugation processes as well as a nanosized architecture for use in photochemical studies.

 Please wait while we load your content...
Please wait while we load your content...