Highly enantioselective Michael reaction employing cycloheptanone and cyclooctanone as nucleophiles†
Abstract
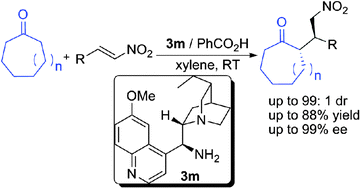
An organocatalytic Michael reaction of cycloheptanone and cyclooctanone with nitrodienes and nitroolefins catalyzed by a hydroquinine-based primary amine catalyst has been accomplished. The corresponding Michael adducts were obtained in good yields (up to 88%) with good to excellent diastereoselectivities (up to >100 : 1) and enantioselectivities (up to >99% ee). The absolute configuration of the Michael product was assigned by TDDFT simulation of the ECD spectrum. And the Michael products can be readily converted into analogues of cycloalkano[b] fused pyrrolidines.

 Please wait while we load your content...
Please wait while we load your content...