HSA carbonylation with methylglyoxal and the binding/release of copper(ii) ions
Abstract
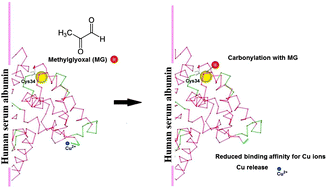
The potential of carbonylation with methylglyoxal to alter HSA's binding affinity for copper(II) ions and its influence on the release of copper(II) ions from copper–HSA complexes were studied. The affinity of HSA to coordinate copper(II) decreased upon carbonylation of the Cys34-SH group. Carbonylation of copper–HSA complexes caused a decrease in Cys34-SH content, conformational changes and the release of copper(II) ions. The ratio between the percentage of reduction in the Cys34-SH group content and the percentage of release of copper(II) from complexes is 2.12 ± 0.28. Because the same ratio (1.96 ± 0.36) was obtained upon oxidation of the Cys34-SH group (with no changes in HSA conformation), the binding/release of copper (II) by HSA depended mainly on the redox state of the Cys34-SH group. The contents of Cys34-SH and HSA-bound copper(II) ions in the diabetic group (0.457 ± 0.081 mol SH per mol HSA, 10.7 ± 0.01 mmol per mol HSA, resp.) were significantly lower (p < 0.01) compared to the control group (0.609 ± 0.027 mol SH per mol HSA; 13.4 ± 0.01 mmol per mol HSA, resp.). Very strong correlations between the values for HSA-SH and glycated haemoglobin, HbA1c, (R = −0.803, p < 0.01), and between the values for the HSA-bound copper(II) content and HSA-SH content (R = 0.841, p < 0.002) were found in the diabetic group. Thus, HSA carbonylation leads to decrease in HSA-SH content and to the impairment of its copper(II) binding capacity that could contribute to further enhancement of oxidative and carbonyl stress in diabetes (as well as in other diseases with carbonyl stress).

 Please wait while we load your content...
Please wait while we load your content...