EPR spectroscopy identifies Met and Lys residues that are essential for the interaction between the CusB N-terminal domain and metallochaperone CusF
Abstract
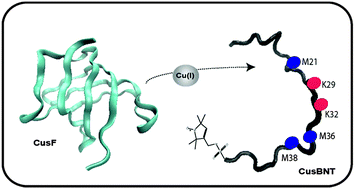
Copper plays a key role in all living organisms by serving as a cofactor for a large variety of proteins and enzymes involved in electron transfer, oxidase and oxygenase activities, and the detoxification of oxygen radicals. Due to its toxicity, a conserved homeostasis mechanism is required. In E. coli, the CusCFBA efflux system is a copper-regulating system and is responsible for transferring Cu(I) and Ag(I) out of the periplasm domain into the extracellular domain. Two of the components of this efflux system, the CusF metallochaperone and the N-terminal domain of CusB, have been thought to play significant roles in the function of this efflux system. Resolving the metal ion transport mechanism through this efflux system is vital for understanding metal- and multidrug-resistant microorganisms. This work explores one aspect of the E. coli resistance mechanism by observing the interaction between the N-terminal domain of CusB and the CusF protein, using electron paramagnetic resonance (EPR) spectroscopy, circular dichroism (CD), and chemical cross-linking. The data summarized here show that M36 and M38 of CusB are important residues for both the Cu(I) coordination to the CusB N-terminal domain and the interaction with CusF, and K32 is essential for the interaction with CusF. In contrast, the K29 residue is less consequential for the interaction with CusF, whereas M21 is mostly important for the proper interaction with CusF.

 Please wait while we load your content...
Please wait while we load your content...