Rhizobium leguminosarum HupE is a highly-specific diffusion facilitator for nickel uptake†
Abstract
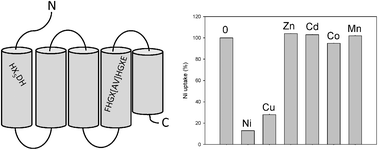
Bacteria require nickel transporters for the synthesis of Ni-containing metalloenzymes in natural, low nickel habitats. In this work we carry out functional and topological characterization of Rhizobium leguminosarum HupE, a nickel permease required for the provision of this element for [NiFe] hydrogenase synthesis. Expression studies in the Escherichia coli nikABCDE mutant strain HYD723 revealed that HupE is a medium-affinity permease (apparent Km 227 ± 21 nM; Vmax 49 ± 21 pmol Ni2+ min−1 mg−1 bacterial dry weight) that functions as an energy-independent diffusion facilitator for the uptake of Ni(II) ions. This Ni2+ transport is not inhibited by similar cations such as Mn2+, Zn2+, or Co2+, but is blocked by Cu2+. Analysis of site-directed HupE mutants allowed the identification of several residues (H36, D42, H43, F69, E90, H130, and E133) that are essential for HupE-mediated Ni uptake in E. coli cells. By using translational fusions to reporter genes we demonstrated the presence of five transmembrane domains with a periplasmic N-terminal domain and a C-terminal domain buried in the lipid bilayer. The periplasmic N-terminal domain contributes to stability and functionality of the protein.
- This article is part of the themed collection: Nickel in biology

 Please wait while we load your content...
Please wait while we load your content...