C1–C2-linker substituted 1,5-naphthyridine analogues of oxabicyclooctane-linked NBTIs as broad-spectrum antibacterial agents (part 7)†
Abstract
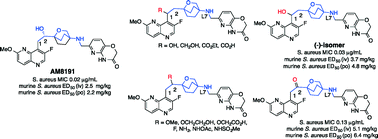
Novel bacterial topoisomerase inhibitors (NBTIs) are a recent class of broad-spectrum antibacterial agents targeting bacterial DNA gyrase and topoisomerase IV at a site distinct from quinolone binding. They are not cross-resistant to known antibiotics and present an excellent opportunity to combat drug-resistant bacteria. We have recently reported a series of oxabicyclooctane-linked inhibitors describing the structure–activity relationship around left-hand-side and right-hand-side moieties. In this report, SAR of the benzylic (C-1) and homobenzylic (C-2) positions of the linker moiety has been described. Single and double substitutions by polar and charged (OH, NH2, CO2H) and non-polar (F, Me) groups indicated that a hydroxy substitution at the benzylic or homobenzylic position is preferred for the potency and spectrum. The C-1,2-dihydroxy group was not effective. Amino substitution at C-2 provides a marginal advantage to the Gram-negative activity. It appears that the α-hydroxy enantiomer was preferred. Despite the beneficial effects of C-1 hydroxy–C-1 alkyl substitution in the tricyclics (particularly for attenuation of hERG), methyl tert-carbinols either at C-1 or C-2 had a detrimental effect on the activity without having much effect on the hERG signal. Mono-hydroxy compounds at C-1 and C-2 showed improved intravenous (ED50 2–4 mg kg−1) and oral (ED50 2–5 mg kg−1) efficacy in a mouse model of bacteremia of S. aureus infection.

 Please wait while we load your content...
Please wait while we load your content...