Novel peptidomimetics related to gonadotropin-releasing hormone (GnRH)†
Abstract
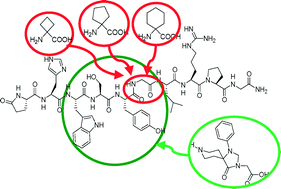
Novel GnRH I and II analogues were designed and synthesized by Solid Phase Peptide Synthesis (SPPS), since GnRH has antiproliferative properties, but poor metabolic stability. To rationalize synthetic difficulties, molecular dynamics simulations were performed, showing the conformational behavior of three derivatives. Among the two series of peptidomimetics (Ie, f and IIe, f were GnRH I and GnRH II analogues, respectively), several compounds (Id–f and IIc–e) showed significant binding affinity. In particular, derivative Ie has an increased metabolic stability with respect to the physiological ligand (Iet1/2 = 3.96 h versus GnRH I t1/2 = 2.63 h).

 Please wait while we load your content...
Please wait while we load your content...