D1-like receptors distinguishing thieno-azecine regioisomers
Abstract
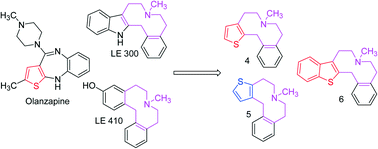
Designing ligands with D1/D5 subtype selectivity is a challenge because of the high identity within the receptor helices. Based on the lead compounds 1–3, the thieno-benzazecine regioisomers 4 and 5 were synthesized and biologically evaluated for their affinity towards the five dopamine receptor subtypes utilizing a radioligand binding affinity technique. Within the D1-like family, compound 4 showed 20 fold selectivity for the D5 subtype over D1 subtype (Ki = 3 nM, D1: 60 nM), while its regioisomer, compound 5 with a reversed thiophene position, prefers the D1 subtype over the D5 subtype (Ki = 4 nM, D5: 15 nM). The benzothieno-benzazecine analog 6 was shown to be one of the few azecine derivatives with high affinity for both the D1- and the D2-like family members in the same order of magnitude (Ki = 1.5 nM for D2 and 1.9 nM for D5). Thorough analysis of the amino acid residues constituting the binding pockets of the target dopamine receptor subtypes revealed that at the D5 receptor, either serine S 6.62 and threonine T 7.33 residues or a water network, stabilized by anionic amino acids could contribute to the selectivity pattern of the synthesized compounds.

 Please wait while we load your content...
Please wait while we load your content...