5-Iodo-2-arylalkylthio-6-aryl pyrimidin-4(3H)-ones as non-nucleoside anti-HBV agents†
Abstract
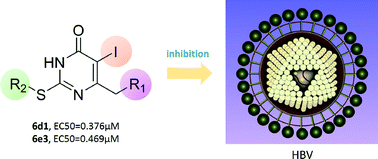
A series of 5-iodo-2-arylalkylthio-6-aryl pyrimidin-4(3H)-ones, which can be considered as S-DABO derivatives, have been synthesized and their antiviral effect on extracellular HBV DNA was evaluated using the HepAD38 cell system. Compounds 6d1 and 6e3 exhibited more potent anti-HBV activity than lamivudine with EC50 values of 0.376 μM and 0.469 μM, respectively. In addition, inhibition of intracellular HBV DNA, pgRNA, HBeAg and HBsAg of compounds 6d1 and 6e3 was examined to initially infer the action mechanism. RT-PCR analysis of pgRNA demonstrated that these new S-DABO analogues could not interfere with HBV transcription. TRFIA analysis revealed that compounds 6d1 and 6e3 effectively reduced the secretion of HBeAg. These results demonstrated that 5-iodo-2-arylalkylthio-6-aryl pyrimidin-4(3H)-ones possess anti-HBV abilities and could be used as potential agents against HBV infection with an additional merit of low cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...