Synthesis, biological evaluation and mechanism study of a class of benzylideneindanone derivatives as novel anticancer agents†
Abstract
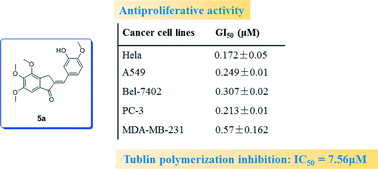
A series of new benzylideneindanone derivatives were designed, synthesized and evaluated as antitumor agents. Structure–activity relationship (SAR) studies showed that derivatives with 4,5,6-trimethoxyl on an indanone moiety displayed good anti-proliferative activities. Especially, compound 5a demonstrated the most potent inhibitory activity, with GI50 values from 0.172 to 0.57 μM for five kinds of cancer

 Please wait while we load your content...
Please wait while we load your content...