Beyond the affinity for protein kinase C: exploring 2-phenyl-3-hydroxypropyl pivalate analogues as C1 domain-targeting ligands†
Abstract
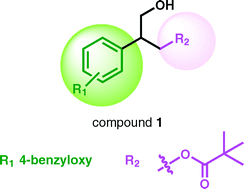
Over the past fifteen years, we reported the design and synthesis of different series of compounds targeting the C1 domain of protein kinase C (PKC) that were based on various templates. Out of the pivalate templates, 2-[4-(benzyloxy)phenyl]-3-hydroxypropyl pivalate (compound 1) emerged as the most potent and promising PKCα ligand, showing a Ki value of 0.7 μM. In the present contribution our efforts are aimed at better understanding which structural modifications of the pivalate template are allowed for its affinity to the C1 domain of PKC to be preserved or increased. To this aim, thirteen novel analogues of 1 were designed and their interaction with the target was evaluated in silico. Designed compounds were then prepared and fully characterized as well as their affinity for the α and δ isoforms of PKC evaluated. Additionally, in order to investigate the role of chirality in the ligand–target interaction, the pure enantiomers of the most interesting PKC ligands were prepared and their affinity for PKC isoforms was determined. Results from our study revealed that: i) the presence of the ester function seems to be essential for the ligand–target interaction; ii) only a few structural modifications at the ester group are allowed for the C1 domain affinity to be preserved; and iii) the [3H]PDBu replacement experiments showed that the C1 domain of PKC does not exhibit enantiopreference for the pure stereoisomers of tested compounds. Altogether our observations provide further insights into the ligand–target interactions of the PKC C1 domain and represent a step-forward in future development of more specific and effective PKC ligands.

 Please wait while we load your content...
Please wait while we load your content...