A novel robust quantitative Förster resonance energy transfer assay for protease SENP2 kinetics determination against its all natural substrates†
Abstract
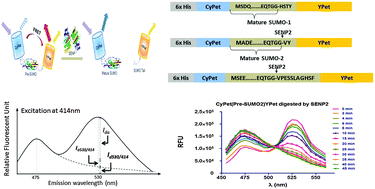
SUMOylation (the process of adding the SUMO [small ubiquitin-like modifier] to substrates) is an important post-translational modification of critical proteins in multiple processes. Sentrin/SUMO-specific proteases (SENPs) act as endopeptidases to process the pre-SUMO or as isopeptidases to deconjugate the SUMO from its substrate. Determining the kinetics of SENPs is important for understanding their activities. Förster resonance energy transfer (FRET) technology has been widely used in biomedical research and is a powerful tool for elucidating protein interactions. In this paper we report a novel quantitative FRET-based protease assay for SENP2 endopeptidase activity that accounts for the self-fluorescent emissions of the donor (CyPet) and the acceptor (YPet). The kinetic parameters, kcat, KM, and catalytic efficiency (kcat/KM) of catalytic domain SENP2 toward pre-SUMO1/2/3, were obtained by this novel design. Although we use SENP2 to demonstrate our method, the general principles of this quantitative FRET-based protease kinetic determination can be readily applied to other proteases.

 Please wait while we load your content...
Please wait while we load your content...