Engineering of a peptide probe for β-amyloid aggregates†
Abstract
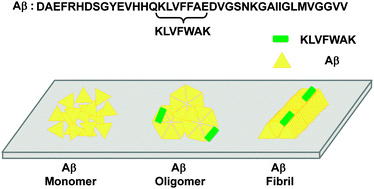
Aggregation of β-amyloid (Aβ) is central to the pathogenesis of Alzheimer's disease (AD). Aβ aggregation produces amyloid assemblies, such as oligomers and fibrils. In contrast to non-toxic Aβ monomers, Aβ oligomers and fibrils can act directly as major toxic agents and indirectly as pools of the toxic entities, respectively. Thus, the detection of Aβ aggregates is of diagnostic interest and should benefit enhanced molecular understanding of AD. Among many molecular platforms, peptide-based ligands hold promise as Aβ probes due to their relative simplicity, ease of optimization and facile conjugation to other molecular contexts. In this regard, Aβ hydrophobic segments (critical in Aβ self-assembly) or variants thereof can serve as lead molecules for Aβ probe development. Unfortunately, the resulting peptides are either highly self-aggregation-prone or their probe potential has not been thoroughly examined. In the present study, we characterized a novel peptide ligand, KLVFWAK, which was created by simple point mutations of an Aβ hydrophobic segment (16KLVFFAE22). We found that KLVFWAK displayed low self-aggregation propensity and was preferentially bound to Aβ oligomers and fibrils relative to Aβ monomers. Interestingly, binding of KLVFWAK to Aβ aggregates occurred at a non-homologous Aβ segment (e.g., Aβ C-terminal domain) rather than the homologous 16KLVFFAE22. We also show that detection of Aβ aggregates during incubation of fresh Aβ was possible with KLVFWAK, further supporting KLVFWAK's high probe potential for Aβ aggregates. In short, this study presents creation of a non-self-aggregating peptide ligand for Aβ aggregates through simple point mutation of an Aβ-derived segment.

 Please wait while we load your content...
Please wait while we load your content...