High-resolution structural characterization of Noxa, an intrinsically disordered protein, by microsecond molecular dynamics simulations
Abstract
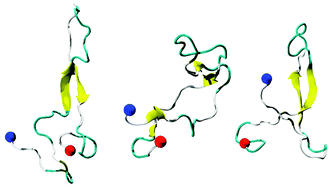
High-resolution characterization of the structure and dynamics of intrinsically disordered proteins (IDPs) remains a challenging task. Consequently, a detailed understanding of the structural and functional features of IDPs remains limited, as very few full-length disordered proteins have been structurally characterized. We have performed microsecond-long molecular dynamics (MD) simulations of Noxa, the smallest member of the large Bcl-2 family of apoptosis regulating proteins, to characterize in atomic-level detail the structural features of a disordered protein. A 2.5 μs MD simulation starting from an unfolded state of the protein revealed the formation of a central antiparallel β-sheet structure flanked by two disordered segments at the N- and C-terminal ends. This topology is in reasonable agreement with protein disorder predictions and available experimental data. We show that this fold plays an essential role in the intracellular function and regulation of Noxa. We demonstrate that unbiased MD simulations in combination with a modern force field reveal structural and functional features of disordered proteins at atomic-level resolution.
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...