Slow ligand-induced conformational switch increases the catalytic rate in Plasmodium falciparum hypoxanthine guanine xanthine phosphoribosyltransferase†
Abstract
P. falciparum (Pf) hypoxanthine guanine xanthine phosphoribosyltransferase (HGXPRT) exhibits a unique mechanism of activation where the enzyme switches from a low activity (unactivated) to a high activity (activated) state upon pre-incubation with substrate/products. Xanthine phosphoribosylation by unactivated PfHGXPRT exhibits a lag phase, the duration of which reduces with an increase in concentration of the enzyme or substrate, PRPP·Mg2+. Activated PfHGXPRT does not display the lag phase and exhibits a ten-fold drop in the Km value for PRPP·Mg2+. These observations suggest the involvement of ligand-mediated oligomerization and conformational changes in the process of activation. The dipeptide Leu–Lys in the PPi binding site of human and T. gondii HG(X)PRT that facilitates PRPP·Mg2+ binding by isomerization from trans to cis conformation is conserved in PfHGXPRT. Free energy calculations using the well-tempered metadynamics technique show the ligand-free enzyme to be more stable when this dipeptide is in the trans conformation than in the cis conformation. The high rotational energy barrier observed for the conformational change from experimental and computational studies permits delineation of the activation mechanism.
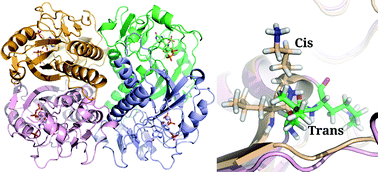
- This article is part of the themed collection: Chemical Biology in Molecular BioSystems

 Please wait while we load your content...
Please wait while we load your content...