Permethrin and its metabolites affect Cu/Zn superoxide conformation: fluorescence and in silico evidences
Abstract
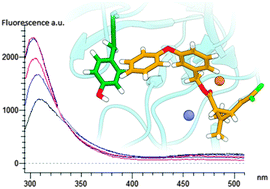
The proclivity of permethrin and its metabolites to affect the structure and activity of Cu/Zn superoxide dismutase (SOD) has been investigated by using intrinsic fluorescence and 8-ANS fluorescence techniques. In silico molecular docking investigations were carried out in order to assess the means of interaction at a molecular level between SOD and the considered ligands. Results show that both, permethrin and its metabolites are able to induce conformational variation on SOD. Permethrin and 3-phenoxybenzyl alcohol metabolite induce a blue shift toward the hydrophobic amino acids Leu-101, Ile-102, Leu-104, Ile-110 and Ile-111, with a significant peak increase. An opposite effect was shown by 3-phenoxy benzaldehyde and 3-phenoxybenzoic acid with a progressive reduction of tyrosine fluorescence emission, without any shift. Computational findings confirm that all the molecules considered have more than one allosteric binding site but none of them interact with SOD at its catalytic Cu/Zn cleft. Moreover, all the binding poses found are very close in binding energy thus demonstrating that there is not only a preferred interaction site but most of them are important due to their relative energy in equilibrium with a population strictly connected to the ligand concentration. In the obtained complexes, all the ligands are involved in many hydrogen bonds through their polar oxygen moieties but due to the presence of a common aromatic hydrophobic core, many hydrophobic interactions are due to the SOD nature rich in apolar amino acids. Furthermore, for each ligand it can be pointed out the presence of a highly populated docked structure with a specific interaction of permethrin and its metabolites with Tyr-108, responsible for changes in fluorescence emission.

 Please wait while we load your content...
Please wait while we load your content...