Polysiloxane layers created by sol–gel and photochemistry: ideal surfaces for rapid, low-cost and high-strength bonding of epoxy components to polydimethylsiloxane†
Abstract
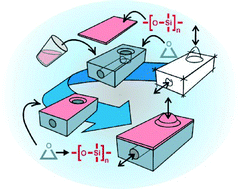
In this article we introduce and compare three techniques for low-cost and rapid bonding of stereolithographically structured epoxy components to polydimethylsiloxane (PDMS). In short, we first create a polysiloxane layer on the epoxy surface via silane surface coupling and polymerization. Afterwards, the modified epoxy surface can be bonded to a PDMS component at room temperature using a handheld corona discharger, which is a commonly used low-cost technique for bonding two PDMS components. Using these methods bonds of desirable strength can be generated within half an hour. Depending on the epoxy resin, we found it necessary to modify the silanization procedure. Therefore, we provide a total of three different silanization techniques that allow bonding of a wide variety of stereolithographically structurable epoxy resins. The first technique is a UV-light induced silanization process which couples a silane that contains an epoxy-ring ((3-glycidoxypropyl)trimethoxysilane (GPTMS)). For surfaces that cannot be modified with this silane we use dimethoxydimethylsilane (DMDMS). This silane can either be coupled to the surface by a sol–gel process or UV-light induced polymerisation. The sol–gel process which is a heat induced surface modification technique results in high bond strengths. Because of the heat which triggers the sol–gel process, this technique is limited to epoxy polymers with high glass transition temperatures. For the majority of stereolithographically structured epoxy resins which typically have glass transition temperatures of around 60 °C the light-induced bonding technique is preferable. For all three techniques we performed DIN EN-conform tensile testing demonstrating maximum bond strengths of up to 350 kPa which is comparable with bond strengths reported for PDMS-to-PDMS bonds. For all bond methods, long-term stability as well as hydrolytic stability was assessed.

 Please wait while we load your content...
Please wait while we load your content...