Microfluidic analysis of extracellular matrix-bFGF crosstalk on primary human myoblast chemoproliferation, chemokinesis, and chemotaxis†
Abstract
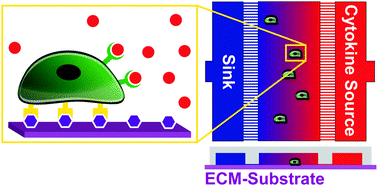
Exposing myoblasts to basic fibroblast growth factor (bFGF), which is released after muscle injury, results in receptor phosphorylation, faster migration, and increased proliferation. These effects occur on time scales that extend across three orders of magnitude (100–103 minutes). Finite element modeling of Transwell assays, which are traditionally used to assess chemotaxis, revealed that the bFGF gradient formed across the membrane pore is short-lived and diminishes 45% within the first minute. Thus, to evaluate bFGF-induced migration over 102 minutes, we employed a microfluidic assay capable of producing a stable, linear concentration gradient to perform single-cell analyses of chemokinesis and chemotaxis. We hypothesized that the composition of the underlying extracellular matrix (ECM) may affect the behavioral response of myoblasts to soluble bFGF, as previous work with other cell types has suggested crosstalk between integrin and fibroblast growth factor (FGF) receptors. Consistent with this notion, we found that bFGF significantly reduced the doubling time of myoblasts cultured on laminin but not fibronectin or collagen. Laminin also promoted significantly faster migration speeds (13.4 μm h−1) than either fibronectin (10.6 μm h−1) or collagen (7.6 μm h−1) without bFGF stimulation. Chemokinesis driven by bFGF further increased migration speed in a strictly additive manner, resulting in an average increase of 2.3 μm h−1 across all ECMs tested. We observed relatively mild chemoattraction (∼67% of myoblast population) in response to bFGF gradients of 3.2 ng mL−1 mm−1 regardless of ECM identity. Thus, while ECM-bFGF crosstalk did impact chemoproliferation, it did not have a significant effect on chemokinesis or chemotaxis. These data suggest that the main physiological effect of bFGF on myoblast migration is chemokinesis and that changes in the surrounding ECM, resulting from aging and/or disease may impact muscle regeneration by altering myoblast migration and proliferation.

 Please wait while we load your content...
Please wait while we load your content...