Tandem Lewis/Brønsted homogeneous acid catalysis: conversion of glucose to 5-hydoxymethylfurfural in an aqueous chromium(iii) chloride and hydrochloric acid solution†
Abstract
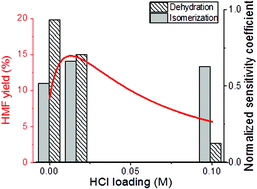
A kinetic model for the tandem conversion of glucose to 5-hydroxymethylfurfural (HMF) through fructose in aqueous CrCl3–HCl solution was developed by analyzing experimental data. We show that the coupling of Lewis and Brønsted acids in a single pot overcomes equilibrium limitations of the glucose–fructose isomerization leading to high glucose conversions and identify conditions that maximize HMF yield. Adjusting the HCl/CrCl3 concentration has a more pronounced effect on HMF yield at constant glucose conversion than that of temperature or CrCl3 concentration. This is attributed to the interactions between HCl and CrCl3 speciation in solution that leads to HMF yield being maximized at moderate HCl concentrations for each CrCl3 concentration. This volcano-like behavior is accompanied with a change in the rate-limiting step from fructose dehydration to glucose isomerization as the concentration of the Brønsted acid increases. The maximum HMF yield in a single aqueous phase is only modest and appears independent of catalysts’ concentrations as long as they are appropriately balanced. However, it can be further maximized in a biphasic system. Our findings are consistent with recent studies in other tandem reactions catalyzed by different catalysts.

 Please wait while we load your content...
Please wait while we load your content...