Application of cyanobacteria for chiral phosphonate synthesis†
Abstract
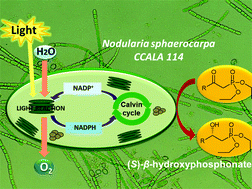
This is the first report on morphologically different strains of cyanobacteria: Arthrospira maxima, Nostoc cf-muscorum and Nodularia sphaerocarpa used for enantioselective bioreduction of selected, structurally different diethyl esters of oxophosphonic acids. The efficiency of the asymmetric hydrogen transfer was strongly dependent on the chemical structure of the substrates. Arthrospira maxima was active only toward diethyl (S)-2-oxopropylphosphonate (20% of yield, 99% of ee), whereas the application of Nostoc cf-muscorum as a biocatalyst allowed diethyl (S)-2-hydroxy-2-phenylethylphosphonate with a high enantiomeric excess (99%) and with 26% conversion degree to be obtained. Employing Nodularia sphaerocarpa led to the most spectacular result – diethyl (S)-2-hydroxy-2-phenylethylphosphonate with a degree of conversion of 99% and an optical purity of 92%. Enantioselective bioconversion of oxophosphonate with an aromatic side group located in the immediate vicinity of the carbonyl functionality was achieved for the first time. Additionally, flow cytometry showed excellent resistance of the cells of Nodularia sphaerocarpa against the examined xenobiotic – 2-oxo-2-phenylethylphosphonate, these cells remain viable at the concentration of 10 mM of the bioconversion substrate compared to the 1 mM described previously for a fungal biocatalyst. The effect of cultivation medium, light source and light cycle (light : dark) on the effectiveness of the biotransformation process was examined.

 Please wait while we load your content...
Please wait while we load your content...