Copper-catalyzed highly efficient oxidative amidation of aldehydes with 2-aminopyridines in an aqueous micellar system†
Abstract
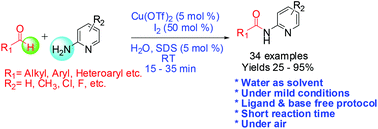
An environmentally benign protocol for the synthesis of N-(pyridine-2-yl)amides from aldehydes and 2-aminopyridines has been developed under mild reaction conditions. This approach requires Cu(OTf)2 as a catalyst, and inexpensive molecular iodine as an oxidant under anionic micellar catalysis in aqueous medium at room temperature.

 Please wait while we load your content...
Please wait while we load your content...