Highly efficient regioselective synthesis of pyrroles via a tandem enamine formation–Michael addition–cyclization sequence under catalyst- and solvent-free conditions†
Abstract
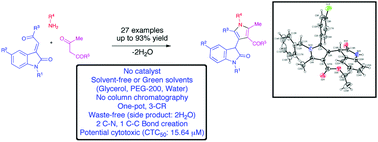
A convenient catalyst-free, three-component procedure was developed for the synthesis of tetrasubstituted pyrroles under solvent-free conditions and in green solvents glycerol, PEG-200 and water. A sequential enamine-formation, Michael addition and intramolecular cyclization of primary amines, 1,3-dicarbonyl compounds and isatin-derived Michael acceptors afforded 3-(1H-pyrrol-3-yl)indolin-2-ones in excellent yields. In this simple one-pot transformation the requirement of column chromatography purification of the products was completely avoided. Besides, the method is highly environmentally benign and atom-economical, and the only side product of this reaction was two molecules of water. A comparative study for the four developed conditions showed that the solvent-free conditions were superior regardless of the nature of the starting materials, and the green solvents were effective for alkyl and benzylamines affording higher yields compared to arylamines. The preliminary in vitro cytotoxic studies of a representative compound against Ehrlich's ascites carcinoma (EAC) tumor cells showed significant activity with a CTC50 value of 15.64 μM.

 Please wait while we load your content...
Please wait while we load your content...