An ionic liquid-based synergistic extraction strategy for rare earths†
Abstract
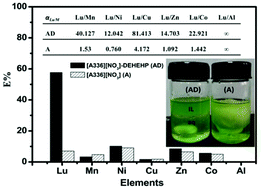
In this work, a novel IL-based synergistic extraction system utilizing the ionic liquid tricaprylmethylammonium nitrate ([A336][NO3]) and the commercial extractant di(2-ethylhexyl) 2-ethylhexyl phosphonate (DEHEHP) was developed for the extraction of rare earth (RE) nitrates. Pr(III) was used as a model RE and the effects of key factors, i.e. the ratio of [A336][NO3] to DEHEHP, the acidity of feed solutions, and the concentration of a salting-out reagent, were systematically studied. Our results demonstrate that the mixture of [A336][NO3] and DEHEHP had an obviously synergistic extraction effect for the extraction of Pr(III). The maximum synergistic enhancement coefficient of 3.44 was attained at XA = 0.4 (v%). Alternatively, a mixture of [A336][Cl] and DEHEHP hardly extracted Pr(III) from chloride media. Moreover, we investigated the Pr(III) extraction mechanism and demonstrated that Pr(III) can be extracted as the neutral complexation species Pr(NO3)3·xDEHEHP and the ion-type species [A336]y·Pr(NO3)3+y. These extraction processes can effectively hamper the release of organic cation-ligands into the aqueous phase. The synergistic extraction effect is mainly derived from the enhanced solubility of the extracted species in the ionic liquid phase. The extraction behaviors of Pr(III) could be properly described by Langmuir and pseudo-second-order rate equations. An increase in temperature was unfavorable for the extraction reaction but greatly improved the extraction rate. Interestingly, the mixed IL extraction system has an obviously synergistic extraction effect for light REs (LREs, La–Eu), but an anti-synergistic effect for heavy REs (HREs, Gd–Lu, Y), thus indicating that our synergistic extraction system is helpful for the separation of LREs from HREs. In addition, the high selectivity between REs and non-REs suggested that the recovery of REs from a complicated high-salt leachate could be highly possible. It demonstrates that the IL-based synergistic extraction strategy developed in this work is promising and sustainable, and as a result the development of an IL-based synergistic extraction process for the recovery of REs is straightforwardly envisaged.

 Please wait while we load your content...
Please wait while we load your content...