Water soluble kraft lignin–acrylic acid copolymer: synthesis and characterization†
Abstract
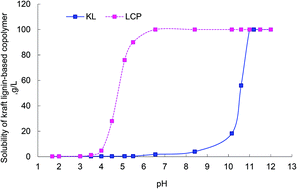
Lignin produced in the kraft pulping process is insoluble in water at neutral pH, which limits its application in industry. In this paper, kraft lignin (KL) was copolymerized with acrylic acid (AA) in an aqueous solution to produce a water soluble lignin-based copolymer. The copolymerization was carried out using K2S2O8–Na2S2O3 as the initiator under alkaline aqueous conditions, and the influence of the reaction parameters, i.e. initiator dosage, reaction time and temperature, mole ratio of acrylic acid to lignin and reaction concentration, on resultant lignin copolymers were investigated. The mechanism of copolymerization of kraft lignin with acrylic acid was also discussed in this work. The resultant lignin copolymer was characterized by Fourier Transform Infrared (FTIR) spectrophotometry and Nuclear Magnetic Resonance (NMR). The successful copolymerization of AA and KL was confirmed by the new absorption peak of carboxyl anions in the FTIR spectrum and new peaks in the 1H-NMR spectrum. At optimal conditions, the charge density and molecular weight of lignin copolymer reached 1.86 meq g−1 and 46 421 g mol−1, respectively, and the solubility of lignin after reaction was increased from 1.80 g L−1 to 100 g L−1 at neutral pH.

 Please wait while we load your content...
Please wait while we load your content...