Ionic liquids: not always innocent solvents for cellulose†
Abstract
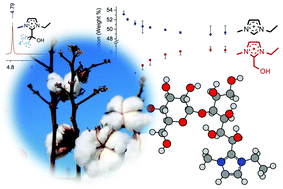
The decomposition pathways of a series of carbohydrates dissolved in carboxylate ionic liquids have been investigated in detail using a broad range of thermal and chromatographic techniques. Mixtures of the carboxylate ionic liquid 1-ethyl-3-methylimidazolium acetate with carbohydrates were found to undergo reaction of the C2 carbon of the imidazolium ring with the aldehyde functionality on the open chain sugar, yielding an imidazolium adduct with a hydroxylated alkyl chain. Subsequently, degradation of the hydroxyalkyl chain occurs by sequential loss of formaldehyde units, to yield a terminal adduct species, 1-ethyl-2-(hydroxymethyl)-3-methylimidazolium acetate. Identities of the final and intermediate adduct species, and the reaction mechanisms connecting adducts, were elucidated by NMR, HPLC and LCMS techniques. Factors affecting the rate and quantity of adduct formation were explored. Changing the ionic liquid cation and anion, the acid number, sugar concentration and temperature influenced the rate of formation and relative quantities of the adduct species. Formation of adducts could not be entirely prevented when employing carboxylate ionic liquids. By contrast, 1-butyl-3-methylimidazolium chloride was identified as an ionic liquid capable of dissolving a significant quantity of cellulose, yet without reacting with carbohydrates.
- This article is part of the themed collections: Celebrating the 150th anniversary of the German Chemical Society and 2015 most accessed Green Chemistry articles

 Please wait while we load your content...
Please wait while we load your content...