Highly efficient construction of a bridged pentacyclic skeleton via a six-component domino reaction under microwave irradiation†
Abstract
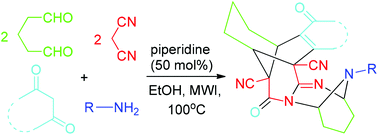
A novel bridged pentacyclic skeleton has been constructed via the six-component domino reaction of glutaraldehyde, malononitrile, cyclic 1,3-dicarbonyl compounds and amines under microwave irradiation. In this one-pot transformation, 11 new bonds and five new rings have been formed. The main advantages of this protocol are short reaction time (within 30 min), practical simplicity, high regioselectivity, benign solvents, atom-economy, and environmental friendliness.

 Please wait while we load your content...
Please wait while we load your content...