Chelate stabilized metal oxides for visible light photocatalyzed water oxidations†
Abstract
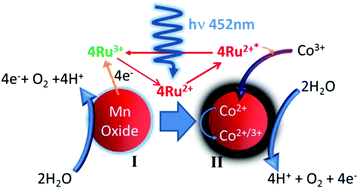
Visible light driven photocatalytic water oxidations were undertaken that compared lactate stabilized molecular and nanoparticle cobalt complexes and calcium manganese oxides as simple mimics of the PSII CaMn4O5 catalyst. Analysis showed chelated cobalt oxides formed as <5 nm particles whilst Ca–Mn and Mn oxides were composed of nanoparticles organized into ∼150 nm spherules. O2 yield, turnover frequency and quantum yields were determined for the chelated materials and compared to sintered CaMn3O6 and Co3O4 counterparts. Results show two distinct stages of O2 generation took place with the chelated calcium manganese oxides, a Ca : Mn molar ratio of 1 : 3 gave highest O2 yield in the initial stage. Significantly, O2 generation at extended reaction time re-occurred without addition of fresh reagents and was determined to be promoted by in situ coating of the lactate metal oxide particles with cobalt captured from the decomposed pentaminecobalt(III)chloride electron acceptor. Time course TEM, XPS and EDX analysis indicated the secondary catalyst accumulated cobalt mainly as Co3O4. These nano-micro particles are readily reused and produced a superior O2 output of 85% maximum theoretical yield. Chelated cobalt catalysts resulted in TOF and O2 yield superior to a laser ablated counterpart, whereas calcium manganese oxide lactates promoted generation of effective recyclable water oxidation catalysts which also minimized toxicity and waste for the photocatalytic system.

 Please wait while we load your content...
Please wait while we load your content...