Catalyst-free one-pot four-component domino reactions in water–PEG-400: highly efficient and convergent approach to thiazoloquinoline scaffolds†‡
Abstract
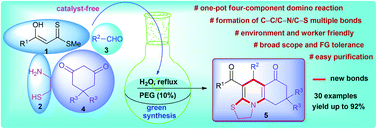
A cost-effective and eco-friendly straightforward synthesis of highly diversified thiazoloquinoline scaffolds is successfully achieved via one-pot four-component cascade reaction utilizing α-enolic dithioesters, cysteamine, aldehydes, and cyclic 1,3-diketones in water–PEG-400. The new efficient domino protocol generates two rings by the concomitant formation of C–C (two), C–N (two), and C–S multiple bonds presumably involving a sequence of N,S-acetal formation, Knoevenagel reaction, aza–ene reaction, imine–enamine/keto–enol tautomerization, and N-cyclization as key steps. The merit of this protocol is highlighted by its easily available and economical starting materials, operational simplicity, efficient utilization of all the reactants, clean reaction profile, simple workup procedure, and tolerance of a wide variety of functional groups.

 Please wait while we load your content...
Please wait while we load your content...