A nanoporous oxygen evolution catalyst synthesized by selective electrochemical etching of perovskite hydroxide CoSn(OH)6 nanocubes†
Abstract
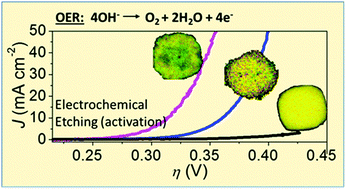
The development of efficient and Earth-abundant oxygen evolution reaction (OER) catalysts is essential for the generation of renewable hydrogen. The synthesis of nanoporous and high-surface-area catalysts remains challenging. Herein, we show that large nanocubes of perovskite hydroxide CoSn(OH)6 can be electrochemically etched to form a highly active OER catalyst, reaching 10 mA cm−2 at an overpotential (η) of only 274 mV. The good catalytic activity was attributed to the formation of hierarchical nanoporous CoOOH particles, which were created by selective dissolution of Sn hydroxides. Crystal defects of oxygen vacancies appear to be important for the formation of active catalysts.

 Please wait while we load your content...
Please wait while we load your content...