Anomalous Jahn–Teller behavior in a manganese-based mixed-phosphate cathode for sodium ion batteries†
Abstract
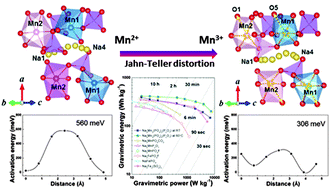
We report a 3.8 V manganese-based mixed-phosphate cathode material for applications in sodium rechargeable batteries; i.e., Na4Mn3(PO4)2(P2O7). This material exhibits a largest Mn2+/Mn3+ redox potential of 3.84 V vs. Na+/Na yet reported for a manganese-based cathode, together with the largest energy density of 416 W h kg−1. We describe first-principles calculations and experimental results which show that three-dimensional Na diffusion pathways with low-activation-energy barriers enable the rapid sodium insertion and extraction at various states of charge of the Na4−xMn3(PO4)2(P2O7) electrode (where x = 0, 1, 3). Furthermore, we show that the sodium ion mobility in this crystal structure is not decreased by the structural changes induced by Jahn–Teller distortion (Mn3+), in contrast to most manganese-based electrodes, rather it is increased due to distortion, which opens up sodium diffusion channels. This feature stabilizes the material, providing high cycle stability and high power performance for sodium rechargeable batteries. The high voltage, large energy density, cycle stability and the use of low-cost Mn give Na4Mn3(PO4)2(P2O7) significant potential for applications as a cathode material for large-scale Na-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...