High-performance symmetric sodium-ion batteries using a new, bipolar O3-type material, Na0.8Ni0.4Ti0.6O2†
Abstract
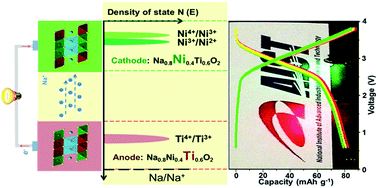
Based on low-cost and rich resources, sodium-ion batteries have been regarded as a promising candidate for next-generation energy storage batteries in the large-scale energy applications of renewable energy and smart grids. However, there are some critical drawbacks limiting its application, such as safety and stability problems. In this work, a stable symmetric sodium-ion battery based on the bipolar, active O3-type material, Na0.8Ni0.4Ti0.6O2, is developed. This bipolar material shows a typical O3-type layered structure, containing two electrochemically active transition metals with redox couples of Ni4+/Ni2+ and Ti4+/Ti3+, respectively. This Na0.8Ni0.4Ti0.6O2-based symmetric cell exhibits a high average voltage of 2.8 V, a reversible discharge capacity of 85 mA h g−1, 75% capacity retention after 150 cycles and good rate capability. This full symmetric cell will greatly contribute to the development of room-temperature sodium-ion batteries with a view towards safety, low cost and long life, and it will stimulate further research on symmetric cells using the same active materials as both cathode and anode.

 Please wait while we load your content...
Please wait while we load your content...