Novel B(Ar′)2(Ar′′) hetero-tri(aryl)boranes: a systematic study of Lewis acidity†
Abstract
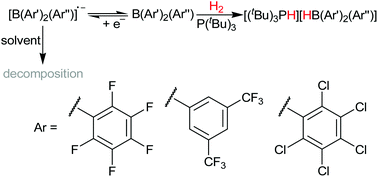
A series of homo- and hetero-tri(aryl)boranes incorporating pentafluorophenyl, 3,5-bis(trifluoromethyl)phenyl, and pentachlorophenyl groups, four of which are novel species, have been studied as the acidic component of frustrated Lewis pairs for the heterolytic cleavage of H2. Under mild conditions eight of these will cleave H2; the rate of cleavage depending on both the electrophilicity of the borane and the steric bulk around the boron atom. Electrochemical studies allow comparisons of the electrophilicity with spectroscopic measurements of Lewis acidity for different series of boranes. Discrepancies in the correlation between these two types of measurements, combined with structural characterisation of each borane, reveal that the twist of the aryl rings with respect to the boron-centred trigonal plane is significant from both a steric and electronic perspective, and is an important consideration in the design of tri(aryl)boranes as Lewis acids.
- This article is part of the themed collection: Main Group Transformations


 Please wait while we load your content...
Please wait while we load your content...