Direct solvothermal preparation of nanostructured fluoride aerogels based on AlF3†
Abstract
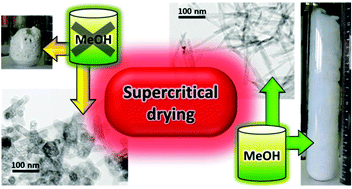
AlF3-based aerogels, a new class of inorganic aerogels, are prepared in a novel direct process that combines fluoride sol–gel synthesis with high temperature supercritical drying. The bulk structure of the solid products depends decisively on the applied solvent(s); very voluminous bulk aerogels are obtained only with MeOH that is used either alone or in combination with some other polar solvents. MeOH acts as a methoxylation agent; and formed methoxy (MeO) species are remarkably stable and deactivate the surface acidic sites. Removal of MeO species under moderate conditions results in catalytically active fluorides with a preserved nanostructure. In preparations with MeOH, preferential growth of anisotropic nanoparticles (nanorods) is the key step that leads to the formation of very open aerogel structures. Another process, dehydration of alcohols, results in some hydroxylation and hydration that lead to the formation of distinctive surface and bulk OH/H2O species. The structure of AlF3-based aerogels is consistent with the hexagonal tungsten bronze (HTB) β-AlF3 although their composition corresponds to a formula AlF3−x(OH, OMe)x·yH2O (x = 0.1 ± 0.05). Some other characteristics of the fluoride nanoparticles, like crystallinity, particle size, and uniformity, can be effectively controlled by the temperature of the solvothermal process. The described methodology allows a controllable preparation of catalytically active fluorides in the form of regularly shaped and uniformly sized nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...