Oxidative photoreactivity of mono-transition-metal functionalized lacunary Keggin anions†
Abstract
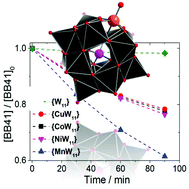
A comparative study investigating the effects of metal substitution on the photocatalytic activity of metal oxide cluster anions is presented. The study shows that metal functionalization can be used to alter the photochemical properties of monolacunary tungstate – based Keggin clusters (α-[TM(H2O)SiW11O39]n− (M = Co2+, Cu2+, Ni2+, Mn2+)). It is demonstrated that the photoactivity for the photooxidation of the model pollutant basic blue 41 is dependent of the type of metal employed and increases in the order Co < Cu < Ni < Mn under aerobic conditions and 390 nm monochromatic irradiation. A significant increase of the reaction rate is observed under aerated conditions compared with de-aerated conditions, suggesting that oxygen serves as a re-oxidant for the reduced clusters. Radical scavenging experiments suggest that the photocatalysis proceeds via formation of hydroxyl radicals.

 Please wait while we load your content...
Please wait while we load your content...