Different topologies in three manganese-μ-azido 1D compounds: magnetic behavior and DFT-quantum Monte Carlo calculations†
Abstract
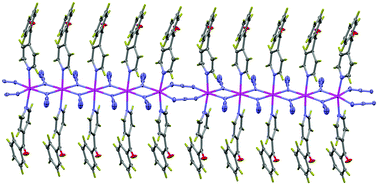
The syntheses and structural characterization of three new monodimensional azido-bridged manganese(II) complexes with empirical formulae [Mn(N3)2(aminopyz)2]n (1), [Mn(N3)2(4-azpy)2]n (2) and [Mn(N3)2(4-Bzpy)2]n (3) (pyz = pyrazine (1,4-diazine)), 4-azpy = 4-azidopyridine and 4-Bzpy = 4-benzoylpyridine) are reported. 1 is a monodimensional compound with double EO azido bridges, 2 is an alternating monodimensional compound with double end-on and double end-to-end azido bridges in the sequence di-EO-di-EE and 3 is a monodimensional compound with double end-on and double end-to-end azido bridges in the sequence di-EO-di-EO-diEO-di-EO-di-EE. The magnetic properties of 1–3 are reported. Periodic DFT calculations were performed to estimate the J values and quantum Monte Carlo simulations were carried out using the calculated J values to check their accuracy in comparison with the experimental magnetic measurements. From this theoretical analysis, two appealing features of the di-EO Mn(II) compounds can be extracted: first, the exchange coupling becomes more ferromagnetic when the Mn–N–Mn bridging angle becomes larger and the spin density of the bridging nitrogen atoms has an opposite sign to that of the Mn(II) centers.

 Please wait while we load your content...
Please wait while we load your content...