Solutions of complex copper salts in low-transition-temperature mixture (LTTM)†
Abstract
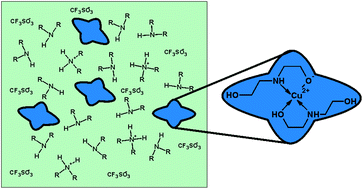
The structure and properties of diethanolamine complexes of copper(II) triflates dissolved in an excess of diethanolamine (DH2) were studied. The copper containing substance was found to be a solution of copper(II) complex salt [Cu2+DH2(DH−)]OTf− in LTTM composition [(DH2)4H+](OTf−), where LTTM = low-transition-temperature mixture, OTf− = triflate anion. According to the EXAFS data, the coordination number of copper(II) atoms in solution does not exceed four. Addition of even negligible amounts of acid significantly changes DH2 volatility and decomposition conditions.

 Please wait while we load your content...
Please wait while we load your content...