Organophosphonate bridged anatase mesocrystals: low temperature crystallization, thermal growth and hydrogen photo-evolution†
Abstract
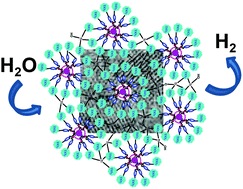
The sol–gel co-condensation of organo-phosphonates to titanium alkoxides enables access to novel organic–inorganic hybrids based on phosphonate-bridged titanium dioxide. In this contribution, we bring new perspectives to the long established sol–gel mineralization of titanium alkoxide species, by harnessing the virtues of the well-designed phosphonate-terminated phosphorus dendrimers as reactive amphiphilic nanoreactor, confined medium and cross-linked template to generate discrete crystalline anatase nanoparticles at low temperature (T = 60 °C). An accurate investigation on several parameters (dendrimer generation, dendrimer-to-titanium alkoxide ratio, precursor reactivity, temperature, solvent nature, salt effect) allows a correlation between the network condensation, the opening porous framework and the crystalline phase formation. The evolution of the dendrimer skeleton upon heat treatment has been deeply monitored by means of 31P NMR, XPS and Raman spectroscopy. Increasing the heteroatom content within a titania network provides the driving force for enhancing their photocatalytic water splitting ability for hydrogen production.

 Please wait while we load your content...
Please wait while we load your content...