Phosphane–ene chemistry: the reactivity of air-stable primary phosphines and their compatibility with the thiol–ene reaction†
Abstract
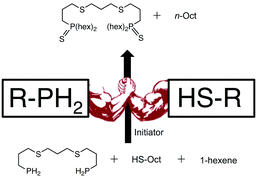
Air-sensitive and air-stable primary phosphines (RPH2) were compared for their ability to undergo photoinitiated phosphane–ene chemistry with 1-hexene. Despite their increased air-stability, the primary phosphines displayed equal to or greater reactivity when compared to air-sensitive alkyl or aryl analogues. The phosphane–ene reaction was also performed in the presence of 1-octanethiol to determine whether thiol–ene and phosphane–ene chemistries could proceed simultaneously. It was determined that the phosphane–ene process takes precedence over thiol–ene as P–H bond conversion was independent of thiol concentration. Tertiary phosphine (R3P) and some secondary phosphine (R2PH) products were found to react with thiols under experimental conditions to create phosphine–sulfides (P–S), but this chemistry only proceeded at low P–H bond concentrations. These results suggests that hydrogen transfer reactions take precedence over P–S formation and demonstrate the unique relationship between phosphane–ene and thiol–ene chemistry.

 Please wait while we load your content...
Please wait while we load your content...