The effect of cluster size on the optical band gap energy of Zn-based metal–organic frameworks†
Abstract
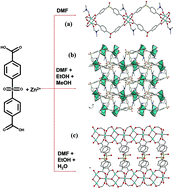
We have synthesized three Metal–Organic Frameworks (MOFs) in which Zn metal ions form the secondary building unit, and 4,4′-sulfonyldibenzoic acid (SDB) serves as the ligand: [[Zn(DMF)(SDB)](DMF), 1, [Zn3(DMF)3(SDB)3](DMF), 2 and [Zn3(OH)2(SDB)2] (DMF)2, 3, where DMF = dimethyl formamide]. Compound 1 contains a paddle-wheel type Zn dimer, compound 2 contains a Zn trimer motif, and 3 contains a one-dimensional Zn–OH–Zn chain. These building units may be considered to be Zn clusters. We have measured and theoretically calculated the band gap energy and by theoretical investigations we found that the cluster size plays an important role in the band gap energy, however additional effects are observed. The larger cluster size corresponds to a larger band gap energy, however the cavity of the trimer based compound (2) traps a solvent molecule that decreases the band gap energy.

 Please wait while we load your content...
Please wait while we load your content...