In situ microcalorimetry study of ZnFe2O4 nanoparticle formation under solvothermal conditions†
Abstract
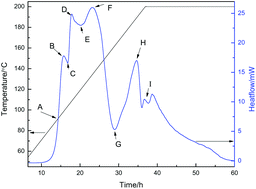
Solvothermal methods have been widely used to synthesize different kinds of materials. However, only little is known about how precursor solutions react to form solid precipitates via this method. In the present study, in situ microcalorimetry is first used to investigate the formation mechanism under solvothermal conditions, where ZnFe2O4 synthesized using a solvothermal method was selected as the model sample. Some novel results are obtained, such as with the experimental temperature increase, (1) the homogeneous solution transforms to a gel containing amorphous Fe2(C2H4O2)3 and ZnC2H4O2, and NaNO3 crystals; (2) the gel dissolves; (3) α-(Fe,Zn)OOH and α-Fe2O3 are synthesized; (4) the α-(Fe,Zn)OOH transforms to α-Fe2O3; (5) Fe2+ is formed at about 159 °C, which acts as a catalyst for the formation of Fe3O4; (6) the Fe3O4 crystals are synthesized at about 200 °C; (7) the Fe3O4 is transformed to the ZnFe2O4 with the help of NO3−, and the reaction was kept at 200 °C for about 20 h. This study shows a facile in situ method for the investigation of reaction processes of solvothermal methods.

 Please wait while we load your content...
Please wait while we load your content...