pH value manipulated phase transition, microstructure evolution and tunable upconversion luminescence in Yb3+–Er3+ codoped LiYF4/YF3 nanoparticles†
Abstract
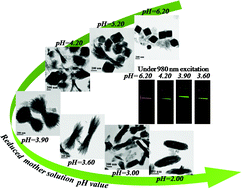
The pH value plays an important role in controlling the crystallization process and microstructure of the final products in the synthesis of nanocrystals with a solvothermal method. This work reported the effect of the mother solution pH value on the precipitation of LiYF4 and YF3 nanoparticles, as well as the microstructure evaluation of YF3 from a bowknot-like to spindle-like shape. Spectroscopy study suggests that there is strong correlation between the upconversion emission properties of the Yb3+–Er3+ couple and the phase and the microstructure of the host. The strongest emissions and lowest red-to-green ratio are observed in the bowknot-like YF3 nanocrystals with the largest open ends. Further spectral investigation indicates that the phase and microstructure dependent upconversion properties are associated with the upconversion efficiency. The present study is of great importance in the design and synthesis of rare earth ion doped nanocrystals with tunable upconversion properties.

 Please wait while we load your content...
Please wait while we load your content...