Frustrated N-heterocyclic carbene–silylium ion Lewis pairs†
Abstract
The reaction of the N-heterocyclic carbene 1,3-di-tert-butyl-4,5-dimethylimidazolin-2-ylidene (1b) with trimethylsilyl iodide, triflate and triflimidate [Me3SiX, X = I, CF3SO3 (OTf), (CF3SO2)2N (NTf2)] by mixing the neat, liquid starting materials afforded the corresponding 2-(trimethylsilyl)imidazolium salts [(1b)SiMe3]X as highly reactive, white crystalline solids. Only the triflimidate (X = NTf2) proved to be stable in solution and could be characterized by means of NMR spectroscopy (in C6D5Br) and X-ray diffraction analysis, whereas dissociation into free 1b and Me3SiOTf was observed for the triflate system, in agreement with the trend derived by DFT calculations; the iodide was too insoluble for characterization. The compounds [(1b)SiMe3]X showed the reactivity expected for frustrated carbene–silylium pairs, and treatment with carbon dioxide, tert-butyl isocyanate and diphenylbutadiyne gave the 1,2-addition products [(1b)CO2SiMe3]X (X = I, OTf, NTf2), [(1b)C(NtBu)OSiMe3]OTf and [(1b)C(Ph)C(SiMe3)CCPh]OTf, respectively. Upon reaction with [AuCl(PPh3)], metal–chloride bond activation was observed, with formation of the cationic gold(I) complexes [(1b)Au(PPh3)]X (X = OTf, NTf2).
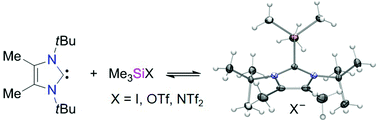

 Please wait while we load your content...
Please wait while we load your content...