N-Alkylfluorenyl-substituted N-heterocyclic carbenes as bimodal pincers†
Abstract
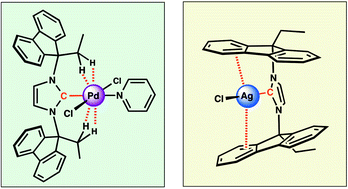
Two N-heterocyclic carbene precursors having their nitrogen atoms substituted by the expanded 9-ethyl-9-fluorenyl group, namely imidazolinium chloride 6 and imidazolium chloride 7, have been synthesized in high yields from fluorenone (1). The key intermediate of their syntheses is the new primary amine 9-ethyl-9-fluorenylamine (3), which was prepared in 75% yield. Both salts were readily converted into the corresponding PEPPSI-type palladium complexes, 8 and 9 (PEPPSI: pyridine-enhanced precatalyst preparation stabilisation and initiation). Despite rotational freedom of the ethylfluorenyl moieties about the N–C(fluorenyl) bond in their cationic precursors, the carbene ligands of the Pd(II)-complexes 8 and 9 both behave as bimodal pincers in solution and in the solid state, the resulting confinement being essentially due to (weak) attractive anagostic interactions between the CH2(fluorenyl) groups and the metal centre. Unlike in 8 and 9, there was no indication for similar anagostic interactions in the imidazolylidene chlorosilver complex 11, which could be obtained from 7. In the solid state, however, 11 adopts a remarkable “open sandwich” structure, with the two alkylfluorenilidene planes η2-bonded to the silver, this constituting a further bimodal pincer-type bonding mode of this ligand class. Complexes 8 and 9 were assessed in Suzuki–Miyaura cross-coupling reactions. The imidazolylidene complex 9 displayed high activity towards unencumbered aryl chlorides. Its activity is comparable to that of the previously reported, highly efficient benzimidazolylidene analogue 10.

 Please wait while we load your content...
Please wait while we load your content...