Color tunable and near white-light emission of two solvent-induced 2D lead(ii) coordination networks based on a rigid ligand 1-tetrazole-4-imidazole-benzene†
Abstract
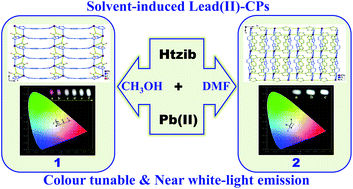
Two new lead(II) coordination polymers, [Pb(NO3)(tzib)]n (1) and [Pb(tzib)2]n (2), were successfully synthesized from the reaction of a rigid ligand 1-tetrazole-4-imidazole-benzene (Htzib) and lead(II) nitrate in different solvents. The obtained polymers have been characterized by single-crystal X-ray diffraction analyses, which show that both polymers feature 2D layer structures. The inorganic anion nitrate in 1 shows a μ2–κO3:κO3 bridging mode to connect adjacent lead ions into a zigzag chain, and then the organic ligands tzib− join the neighboring chains into a 2D layer by a μ3–κN1:κN2:κN6 connection mode. In 2, there are two different bridging modes of the tzib− ligand: μ3–κN1:κN2:κN6 and μ3–κN1:κN6 to coordinate the lead ions into a 2D layer structure. Interestingly, both polymers displayed broadband emissions covering the entire visible spectra, which could be tunable to near white-light emission by varying excitation wavelengths.

 Please wait while we load your content...
Please wait while we load your content...