Zinc(ii)-methimazole complexes: synthesis and reactivity†
Abstract
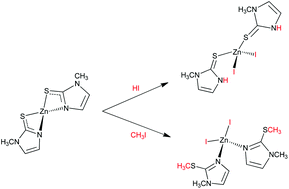
The tetrahedral S-coordinated complex [Zn(MeImHS)4](ClO4)2, synthesised from the reaction of [Zn(ClO4)2] with methimazole (1-methyl-3H-imidazole-2-thione, MeImHS), reacts with triethylamine to yield the homoleptic complex [Zn(MeImS)2] (MeImS = anion methimazole). ESI-MS and MAS 13C-NMR experiments supported MeImS acting as a (N,S)-chelating ligand. The DFT-optimised structure of [Zn(MeImS)2] is also reported and the main bond lengths compared to those of related Zn-methimazole complexes. The complex [Zn(MeImS)2] reacts under mild conditions with methyl iodide and separates the novel complex [Zn(MeImSMe)2I2] (MeImSMe = S-methylmethimazole). X-ray diffraction analysis of the complex shows a ZnI2N2 core, with the methyl thioethers uncoordinated to zinc. Conversely, the reaction of [Zn(MeImS)2] with hydroiodic acid led to the formation of the complex [Zn(MeImHS)2I2] having a ZnI2S2 core with the neutral methimazole units S-coordinating the metal centre. The Zn-coordinated methimazole can markedly modify the coordination environment when changing from its thione to thionate form and vice versa. The study of the interaction of the drug methimazole with the complex [Zn(MeIm)4]2+ (MeIm = 1-methylimidazole) – as a model for Zn-enzymes containing a N4 donor set from histidine residues – shows that methimazole displaces only one of the coordinated MeIm molecules; the formation constant of the mixed complex [Zn(MeIm)3(MeImHS)]2+ was determined.

 Please wait while we load your content...
Please wait while we load your content...