Relationship between crystal structure and thermo-mechanical properties of kaolinite clay: beyond standard density functional theory
Abstract
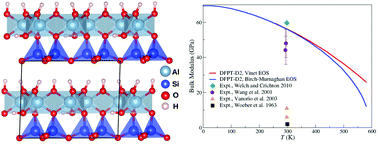
The structural, mechanical and thermodynamic properties of 1 : 1 layered dioctahedral kaolinite clay, with ideal Al2Si2O5(OH)4 stoichiometry, were investigated using density functional theory corrected for dispersion interactions (DFT-D2). The bulk moduli of 56.2 and 56.0 GPa predicted at 298 K using the Vinet and Birch–Murnaghan equations of state, respectively, are in good agreement with the recent experimental value of 59.7 GPa reported for well-crystallized samples. The isobaric heat capacity computed for uniaxial deformation of kaolinite along the stacking direction reproduces calorimetric data within 0.7–3.0% from room temperature up to its thermal stability limit.

 Please wait while we load your content...
Please wait while we load your content...